Welcome to "Save Our Seeds"
‘Save Our Seeds’ (SOS) started as a European initiative in favor of the purity of seeds against genetically modified organisms (GMO) . The initiative was created in 2002 by the Foundation of Future Farming and since then advocates for a zero tolerance for contamination of seeds. Due to new developments in genetic engineering linked to the advent of CRISPR/Cas9, Save Our Seeds enlarged its focus and now also advocates for a GMO free nature.
Hundreds of organizations and some thousand citizens of the EU have become affiliated with Save Our Seeds’ many activities. Its projects strive to keep nature and agriculture free from genetic engineering and promote organic agriculture, biodiversity and food sovereignty.
SOS organizes the yearly GMO Free Regions conference, co-ordinates the European Stop Gene Drive Campaign, the Bantam Mais action and is co-publisher of the Informationsdienst Gentechnik (GE Info Service). SOS was involved in the creation of the Weltagrarbericht (World Agriculture Report) and has shared its findings all over Germany. Together with many other organizations, SOS is responsible for the campaign “Meine Landwirtschaft – Unsere Wahl” (My Agriculture, Our Choice), engaged with the realignment of European agricultural policy after 2013.
With its campaigns and initiatives, SOS networks with different organizations, companies, politicians, scientists, farmers, and interested citizens; and wishes to lead a productive debate towards sustainable change.
Daring Complexity - Cultivating Diversity

The "Colour of Science" is a rather intimate, bilingual congress that brings together scientists and practitioners every 10 years to jointly examine and discuss future topics from different angles. The topic of the third edition is "Daring complexity - cultivating diversity".
The complicated nature of the world we live in escapes nobody's notice. How complex it is, on the other hand, seems worth a closer look. Where did science have its eyes? How can it be that the mother of all diversity of life on this planet, the microbiome, is still largely unexplored? As a scientific discipline in its own right, microbiome research is just 25 years old. Only a tiny fraction of the microbes and viruses, fungi and algae that make up the global microbiome have been mapped so far. Much less is known about the complex network of relationships in these wild communities and about their incredible creativity, adaptability and resilience.
The fact that we humans ourselves harbour more foreign DNA than our own and are entire ecosystems of interacting microorganisms has recently become common knowledge, especially in circles interested in medicine and nutritional science. However, the fact that every plant and every animal is such a holobiont, a symbiotic work of art that has evolved over millions of years and interacts with other holobionts, is still far from being the foundation of our agricultural and nutritional culture. The understanding of this "biological intelligence", its common basis of communication and evolutionary principles, which go far beyond Darwin's findings, is still in its infancy. Of course, it is all connected, but how? We are only just beginning to realise this. You might almost think that such complexity defies male inventive genius and classical scientific evidence through the precise isolation of individual factors.
The benefits that modern agriculture, horticulture and food production have derived from the targeted cultivation of the interplay between plants, animals and microorganisms have been correspondingly modest to date, despite thousands of years of traditional and indigenous groundwork. Against this background, we will discuss practical experiences with intercropping in Kassel and China, in Mexico and Münster, Hesse and Spain, Bavaria and Andra Pradesh, micro and macro. Post-industrial, agro-ecological agriculture and horticulture, including forestry, which is still developing in innovative niches in Europe, has already taken on large-scale dimensions in the two biggest and most tradition-rich agricultural nations in the world, India and China.
A special highlight is therefore the public evening lecture by Vijay Kumar Thallam, the initiator of the Andra Pradesh Community Managed Natural Farming Programme, probably the largest agro-ecological conversion programme in the world based on intensive intercropping and microbiomic techniques, involving one million farms.
Finally, we look at how biological and machine intelligence are meeting. The focus will be on experiences with digital support of agroecological practices on the one hand and perspectives of digital control and appropriation of life on the other.
Please register here if you would like to embark on the adventure of the colour of research from Friday 15 March from 1 pm to Saturday 16 March at 4 pm. In order to form networks between speakers and participants, also in smaller circles of workshops and debates, while eating and drinking together, participation is only possible in person. You can find the programme here. The conference will be bilingual and simultaneously translated into German and English.
EP: narrow vote for GM deregulation
On 7 February, the European Parliament voted by a narrower margin than last feared with a majority of Christian Democrats, Liberals and the more or less extreme Right in favour of the EU Commission's deregulation proposal regarding new genetic engineering techniques. In some areas the parliamentarians want to go even further than the Commission. Their definition of genetic engineering constructs that no longer require safety testing is even more adventurous than that of the Commission. On the other hand the Parliament demands labelling and traceability for all new GMOs, much to the annoyance of the industry. The good news: The representatives of the governments were unable to agree on a common position on the regulation on the same day. Here are Save Our Seed's comments:
'Today's vote by the European Parliament in favour of deregulating the EU's existing GMO legislation was sad news for Europe's agriculture and environment, for the freedom of choice of Europe's consumers, for the precautionary principle and for an enlightened and critical approach to new technologies and the respectful treatment of different forms of cultivation and nutrition. However, today's surprisingly narrow and sometimes contradictory vote in the Parliament is not the end of the debate on honesty, precaution and transparency in the use of genetic engineering on Europe's fields and plates. It has only just begun in the wider public arena.
With a narrow majority, the EP today called for all new genetically engineered products to be labelled. This is a serious setback for the otherwise successful campaign of the industry lobby, which had previously equated labelling and thus freedom of choice for consumers with a ban on the marketing of their products. Parliament's demand to make the new genetically engineered products traceable and to allow for banning them should doubts arise about their safety is also not what the initiators of the deregulation had in mind.
However, the good news of the day is that the EU member states in their Council of Permanent Representatives did not agree on a common position on the Commission's deregulation proposal. This is a serious blow to the industry and its allied anti-ecological front of Christian Democrats, Liberals and the more or less extreme right. The decision will probably only be made after the European elections and the summer break.
Meanwhile, the increasingly clear scientific criticism of the Commission's and Parliament's concept will deepen. The questionable promise to prevent the patenting of genetically engineered seeds will be legally checked. There will be time to thoroughly reconsider the actual risks and possible consequences of complete deregulation. During the election campaign, millions of voters will be able to scrutinise the position of their MPs. Despite the disappointment of today's narrow vote, there is still hope.
After all, today has made it clear that not only the majority of European citizens, but also a strong minority in their parliament continues to stand up for freedom of choice, precaution and respect for farmers and their diverse cultivation methods. We will do our best to ensure that reason and understanding in the democratic handling of new genetic engineering techniques ultimately succeed.
GMO Free Europe 2023

In September 2023 more than 200 critical spirits of the genetic engineering debate across Europe met for the 10th GMO Free Europe Conference at the European Parliament in Brussels and strongly criticized the legislative proposal of the EU Commission to deregulate almost all future GMOs - no risk assessment, no individual approval, no more labeling! The programm and proceedings can be found on the conference webseite
A new GMO narrative
On July 5th the European Commission has released a proposal for a deregulation of so called New Genetic Engineering Technologies, namely CRISPR-Cas, that would reframe the European Unions precautionary legislation on GMOs in food and agriculture. In a longer essay Save Our Seeds co-ordinator Benny Haerlin looks at the spirit and the implications.
Adding, removing or swapping individual base pairs in the genome can make a huge difference to the functioning of a cell and the properties of an entire organism. It’s a bit like Johann Sebastian Bach’s definition of skilful piano playing: you just have to press the right key at the right time. It’s that simple, and yet also that difficult. Therein lies the appeal of genetic engineering, but also its risk.
In a draft new regulation on genetic engineering the European Commission is now proposing that plants that have been genetically modified at up to twenty different sites of the genome should nevertheless be “considered equivalent to conventional plants”. At these twenty sites, any number of base pairs (nucleotides) may be deleted or inverted, up to 20 base pairs may be completely altered or replaced, any length of contiguous DNA sequences may be replaced by related sequences, and any other alteration of any kind may be made that already occurs in any plant that can be crossed directly with the genetically modified plant or via intermediate steps.
No individual risk assessment and approval would be required for those GMOs and they would no longer have to be traceable nor be labelled as GMOs. In short, this would be the end of the precautionary and transparent genetic engineering legislation as we know it and that has been in place since 1990 in various EU directives and regulations.
For this new type of genetic engineering, no longer to be treated as genetic engineering, the Commission has introduced the term “new genomic techniques”. This primarily refers to the CRISPR-Cas technology, also known as “gene scissors”, which is used to create a double break in the DNA at precisely definable points of the genome. As this break is repaired by the cell, the DNA can be specifically altered. Single or multiple base pairs can be rewritten or removed and longer DNA sequences can be silenced or inserted at the break site. In order to carry out such “editing” of the genetic material, the bacterial DNA of the CRISPR-Cas enzyme together with the RNA search mechanism is first introduced into the cell using classical methods of genetic engineering. That bacterial DNA is then removed again if possible.
A new story about genetic engineering
This “genome editing“, according to the central justification for the proposed re-evaluation, is merely creating “targeted mutations” that could also arise “naturally” through conventional breeding. Associated risks to the environment and human health are therefore no higher than the products of conventional breeding. Moreover, they cannot even be reliably distinguished from these. In contrast to “transgenic” organisms, into which “foreign” DNA is inserted, this is “cisgenesis“, which only inserts copies of genetic material from related plants already available in the “breeder’s gene pool ” of these plants wherever in the world. Should the DNA copy not exactly be the same, but already have been “rearranged” a little, for example from different DNA sequences occurring somewhere in the “breeders’ gene pool“, this is “intragenesis“, which is also to be treated as equivalent to conventional plants.
The new dogma derived from this is that genetically modified plants, which were produced by “targeted mutagenesis” and do not contain “foreign” DNA in the broadest sense, cannot pose any different hazard than conventionally bred ones.
What the EU Commission is presenting here is a new narrative, an alternative truth about what genetic engineering means and how it works. Its basic concepts were first developed over 20 years ago by scientists at Wageningen University, with moderate success at the time.
The impetus for this came from ethical discussions with two Christian parties about the integrity of organisms, which might be less violated by genetic modifications with one’s own genetic material than by “foreign” genetic material. Attempts by the Dutch parliament to introduce cisgenetics, intragenetics and the breeder’s pool into European legislation initially failed. However, since CRISPR-Cas has reinvigorated the somewhat flagging imagination of the genetic engineering industry, this narrative was again being massively pushed: “This is not really genetic engineering at all but almost natural mutagenesis”.
Then, in 2018, the European Court of Justice stated in a landmark ruling that all new genetic engineering methods (CRISPR-Cas, Talen, zinc finger nucleases etc.) fall under the EU’s prevailing genetic engineering legislation and may even be riskier than previous transgenic methods. Since then, a multi-million dollar industry lobby and a rather small department of the Directorate-General for Health at the EU Commission have been working to change the legislation upon which the ECJ had ruled: If you can’t change the ruling you must change the law. The European Food Safety Authority (EFSA) was tasked with comparing the risks of these different new “dutch” categories of genetic engineering, thus giving them higher scientific consecration.
If you search the web for scientific literature on cisgenesis, intragenesis or the “breeders gene pool”, you will only find these terms in connection with the allegedly outdated genetic engineering legislation and approval practice in Europe. The narrative of risky transgenes and harmless cis- and intragenes, of brutal genetic manipulations of the past and precise and targeted mutations of the future, offers politicians and companies a way to present their commitment to deregulation of genetic engineering not as a political change of heart, but as the latest scientific discovery.
There are many points in the deregulation proposal where people sufficiently savvy in genetic engineering and the history of the GMO debate can only draw one conclusion: Here, people who have long considered the use of genetic engineering in breeding to be harmless and the EU’s current GM legislation to be completely excessive have concocted a fairy tale for politicians and the general public to allow a speedy exit from the whole “humbug”. “Genome-edited” plants are the entry point for this. The European Food Safety Authority (EFSA) is already working on the re-evaluation of GM micro-organisms.
Would you like something more complex?
However, this narrative of the good and the foreign genes and of what is natural and what is not contradicts many recent insights of molecular biology. Here, a picture is gaining ground that cells and organisms read and combine individual DNA sequences according to rules that are not simply “programmed” into the DNA itself.
The discovery by the Human Genome Project 20 years ago that the human body produces over 100,000 different proteins with only about 25,000 so-called genes, i.e. coding DNA sequences, was a first blow to our picture of the so-called blueprint of life (DNA makes RNA makes protein and there is no way back).
Since then, a multitude of discoveries in epigenetics has put the naïve image of DNA as a programme code into perspective. These include the diverse functions of RNA as well as the complex role of those 99 percent of DNA that do not code for proteins, and were therefore once dismissed as “junk DNA”. It is fascinating: the further science advances into these areas of knowledge, the more complex the picture becomes. CRISPR-Cas technology, which easily enables the targeted “silencing” of individual gene-sequences, provides researchers with an enormously powerful new tool to dig deeper into these complexities.
Highly simplified, one could perhaps compare DNA with a kind of hard wiring, which is used by a still only very incompletely understood “software” of the cell in different states and developmental stages as well as under different environmental conditions. Intervening directly in the DNA in ignorance and bypassing the natural rules and control mechanisms of heredity can bring unexpected risks and side effects. Such a depth of intervention, according to the basic thinking of the EU’s precautionary GM legislation so far, requires special caution and risk assessments. Our experience with such interventions and their direct and indirect, short- and long-term effects is still extremely limited.
Unfortunately, this is where the story of the tremendous precision of the “new genomic technology” ends. Only once you understand the “program” and interrelationships you are directly or indirectly interfering with can you claim real precision. Anyone who can at best state probabilities with which certain changes to individual DNA sequences, no matter how precise, will produce altered characteristics, is still a long way from true causality and reliable assessment of unintended and unwanted effects. Even a blow struck with extreme precision remains but a blow in the air.
Inconsistencies
There are a number of scientific and technical objections to the narrative of targeted mutation and its tremendous precision. Among them is the fact that CRISPR-Cas enzymes can be very precisely targeted to a specific sequence of base pairs for which their RNA “nose” is looking. But knowing the one site of your choice to cause a targeted double-strand break in the helix does not exclude the possibility of many other identical or deceptively similar sequences elsewhere in the genome. The literature on unintended and unforeseen consequences of using CRISPR-Cas for medical purposes is overwhelming. There is no reason to assume that the lists would be smaller in plants, if there was enough energy and funding to search for such unintended breaks.
For example, there was a recent description of an effect of double-strand breaks in plants called “chromothripsis”, in which parts of the affected chromosome break off with massive consequences that are, however, not always easily recognised. This effect had already been known for some time in mammalian and human cells.
The fact that CRISPR-Cas causes breaks even in regions of the genome that are naturally particularly well protected against random mutations strongly challenges the claim that targeted mutations are actually just more harmless and precise variants of what happens all the time in nature.
Last but not least, the special mechanism of CRISPR-Cas to change not only one, but all sites of a certain DNA sequence in the genome at the same time is an effect that can almost certainly be ruled out with natural mutations. It is also a particular strength of the technique: it could be possible to drive out the allergenic gluten production in wheat with its four to six sets of chromosomes and correspondingly many copies of individual genes.
Finally there is this enigmatic idea of George Church, a pioneer and enfant terrible of genetic engineering, wo wants to revive the Tasmanian tiger or the mammoth with his company Collosal. Piece by piece, he wants to string together one CRISPR modification after the next to bridge the 0.4% difference between the genome of the mammoth and that of the Asian elephant. For less ambitious plant breeding goals, the systematic stringing together of individual “targeted mutations” is not necessarily a mammoth task. Entire DNA sequences designed on the screen can be transferred step by step to an organism; if it pleases the EU Commission, also in individual steps of 20 times 20 changes each, which can then be included in the breeder’s pool and made the basis for further steps.
Don’t look, don’t see
Perhaps the most worrying proposal of the EU Commission is the completely new approach to risk assessment and evaluation of new genetic engineering. In the future, the question of what kind of GMO is involved will no longer be examined in terms of the actual organism, but only in terms of the inventive concept of this GMO. The manufacturers inform the authorities of the intended modifications and the examiners examine within 30 days, on the basis of the files, whether in their opinion these modifications can also be produced using conventional breeding techniques or whether they are of a cisgenetic or intragenetic nature.
A concrete analysis and appreciation of unintended changes is no longer provided for. This is reminiscent of the famous drunk who looks for the lost set of keys under the lantern because that is the only place where there is light. Risks and side effects, on the other hand, experience teaches us, should be sought precisely where we do not expect them.
Speaking of the bunch of keys: The puzzling number of a maximum of 20 nucleotides that are now supposed to pass as a “natural mutation” originates – according to scientists involved – from a study of naturally occurring genetic differences in 80 plants of the same plant species (thale cress, something like the laboratory mouse of plant geneticists) from 2011. At that time, the test method used did not allow for longer sequences to be recognised just as reliably, much to the regret of the scientists today, who would prefer an even higher number. So the odd number 20 seems to be primarily due to the limitations of the test methods at the time.
Which path do we want to take?
There is plenty to argue, mock and philosophise about. In the course of the coming years, we will possibly experience breath-taking shocks to our previous ideas of genetics and epigenetics, of DNA and microbiology, which clearly go beyond those of the past decades. Let’s just think of the revolutionary findings about the microbiome that are gaining public acceptance, or the possibilities of calculating the three-dimensional folding of proteins in minutes instead of years by means of so-called artificial intelligence. Computers do this with self-learning programmes whose logic even their inventors can no longer comprehend. Whether this excites or frightens us, we should prepare ourselves for ground-breaking new insights and also for potentially disruptive applications in agriculture and nutrition.
In view of the damage we are currently inflicting on nature in this area, there is no question that fundamental changes are desperately needed. There is also no question that we cannot afford the herbicide-tolerant monocultures of Bayer, Syngenta and Corteva, or their continuation by other means. The first priority must be to get out of the industrial agriculture of the last century, out of its over-fertilisation and poisoning, inefficient over-production, waste and displacement of peasant livelihoods and production of food of ever lower quality. To miss this agro-ecological transformation would not only be risky, but certainly a mortal danger.
Where there is agreement on this agro-ecological turnaround, we should talk about appropriate technologies, priorities and time perspectives in research and development with a sense of proportion and reason, striving to build mutual trust. It is always about complex and diverse systems, not individual products, with which we can best master the various challenges of climate change and species extinction, soil fertility and water management in their respective regional and ecological contexts. We also need to talk about the ecological, health and social controllability and impact of technologies and development paths beyond the technical risks in the narrower sense.
Given the current state of knowledge on the one hand and the enormous speed of technical developments on the other, they can hardly be reduced to the analysis of individual molecular biological facts. The para-alchemistic and dodgy definitions of cisgenesis, intragenesis and breeders’ gene pool not only contradict the requirements of evidence-based and sound science. They also distract from the real challenges, dangers and risks.
The decreasing costs of both the CRISPR-Cas technique and of the sequencing of genomes and their digital processing will play a significant role in the likelihood of technical errors and abuse. Forgoing specific risk assessment and orderly approval, traceability, monitoring and labelling for these products, obviously defies common sense, especially in the current phase of gold rush sentiment. Deregulation in times of exploding technological innovation with uncertain results is the opposite of precaution and prudence. Every politician and every member of a public administration should understand this and could even have Elon Musk and other proponents of artificial intelligence explain it to them.
The New Seed Order
Social control and best use of the innovation potential in agriculture in Europe and the world, also includes the question of who will own the new, but also old technological potential in the future. Deregulation of genetic engineering in conjunction with the greed for “intellectual property” of seeds and individual genetic traits may result in a dangerous combination. Whatever is developed by means of CRISPR-Cas and similar genetic techniques no longer falls under the current European patent law’s fundamental ban on patenting “essentially biological processes for the production of plants and animals” (conventional breeding, for example) and their products. CRISPR-Cas would therefore open the door to patenting seeds or individual traits and no longer “only” under plant variety protection. It is not the only path that the patent lawyers of Bayer, Corteva, Syngenta & Co are currently taking, but it is the easiest.
The key difference: with protected varieties, breeders can continue to develop new varieties without requiring the consent of the variety holder. Farmers can obtain and optimise their own seed from these varieties. With patented seed, on the other hand, nothing happens without the consent and licence of the patent holder. This applies – and this is where the labelling and identifiability of GMOs comes into play – even if the patented trait happens to cross into breeding or planting material: it remains the exclusive property of the patent holder.
At a time when the adaptation and development of new varieties is becoming particularly urgent due to climate change and species loss, the “crisperisation” of breeding would leave the field to an even smaller and more exclusive circle of companies and their law firms. The remnants of “open source”, which are anchored in plant variety rights, would thus be killed off. For every breeding company, the temptation and soon also the competitive pressure would be great to attach a “targeted mutation” to new varieties in order to deny customers the possibility of reproducing them and competitors the use of the genetic material. The entire seed market would thus be subjected to a new world order in a relatively short time.
The consequences of this can already be seen today in America, where small and medium-sized breeding companies have practically disappeared. The preservation and further development of perhaps the most important heritage of humankind, whose diversity has been getting smaller and smaller for decades, would finally degenerate into the exclusive technology of a few seed oligarchs; the same ones, incidentally, who fight the labelling and traceability of these new genetic engineering products with the argument that they are practically indistinguishable from conventionally bred varieties. When it comes to their patents, they will certainly find ways and means to do so.
Practical precaution
Since breeding and genetic engineering of plants always starts at the germ line, i.e. produces self-replicating organisms whose characteristics can also cross-pollinate and spread to related species in nature, precaution is required. We should investigate and assess possible risks in advance. It should be possible to reverse the intervention as far as possible in an emergency, and the released GMOs should be clearly identifiable for this purpose. This is technically not a problem if the producers of the GMO cooperate and submit a test as part of the authorisation dossier, as they have done up to now. It is not disclosure that becomes more difficult, only concealment that becomes somewhat easier.
But the question that is now being debated in the possible deregulation of genetic engineering is a different one: How much respect do we sensibly have for the new possibilities of manipulation that are emerging and for the big picture that we have by no means grasped, into which we intervene? And how much caution do we need to exercise when dealing with the human capacity for innovation and the resulting power relations?
Is it really sensible and necessary to offer the genetic engineering industry, which with all due respect has not yet made a convincing contribution to the ecological transformation and sustainability of food and agriculture, short-term investment incentives through extensive deregulation of the current safety and transparency regulations? Are such advance laurels justified for an industry whose only economic success in the last 30 years has been a system of herbicide-tolerant and insect-poisonous plants that causes enormous environmental and health damage worldwide?
Respect and honesty
Why don’t they first modestly substantiate at least some of their full-bodied promises with cleanly developed products and concepts, in compliance with the applicable regulations? What are they afraid of? Why should it no longer say what’s in it? Those who make such demands make us suspicious.
Furthermore, we should, as is currently the case, respect people who reject this form of dealing with nature in their own fields, gardens and plates for whatever reason – regardless of whether they are a majority or a minority. For this, GMOs and their products must be labelled.
Not least in order not to deal a death blow to organic farming, which excludes the use of genetic engineering. The EU Commission’s proposal continues to affirm the ban on the use of genetic engineering in organic farming. However, it leaves the liability for this exclusively to the organic sector and the regulation of coexistence, which is difficult to imagine, to the member states. And it even ties a hand behind their backs: The hitherto possible national or regional restrictions or even bans on the cultivation of GMOs are categorically excluded for the new GMOs, which can no longer be traced!
Those who make a “ban on genetic engineering” in Europe out of these rules, especially out of the obligation to label, should rethink their relationship to the right to self-determination and freedom of choice of producers and consumers. The attempt to end the controversy on this issue by withholding crucial information from visibly interested citizens in the future, according to the motto “science has determined that you are not interested in this”, is patronising and echoes force feeding.
The whole difference, a single wrong key at the wrong time can make on the political keyboard was recently demonstrated at a hearing in the German Bundestag by liberal MP Ingo Bodke, who explained that targeted mutagenesis was achieved with the help of CRISPR-Cash. He sure has a point, though probably not precisely the one he had targeted.
The EU Commission’s proposal can still fail in the legislature of the European Parliament and the Council of Ministers. Politicians, unlike the Commission, are not only exposed to pressure from industry and the lobby of interested scientists and technicians, which it has masterfully orchestrated in this case, especially in times of looming elections. They also have to adjust to the mood of their own electorate. This will determine whether Wednesday, 5 July 2023 will go down in the history of the EU as the regrettable beginning of the end of precaution and respect for the basis of our agriculture and nutrition, or merely as one of those days the EU Commission made a proposal that was not really well thought out.
300,000 EU citizens call to Stop Gene Drives
Almost 300,000 EU citizens called upon EU environment ministers to demand a global gene drive moratorium at the UN Biodiversity Convention conference in December 2022. Here, the petition with their signatures was handed over to the German Minister of the Environment, Steffi Lemke, in Berlin. Lemke took a clear stance and promised to stand up for the precautionary principle with regard to gene drives at the EU Conference of Ministers of the Environment. She encouraged campaigner Mareike Imken to continue working on the issue, as the debate on the use of gene drives would not end the UN Conference of Parties. Indeed, no reference was made to Gene Drives in the Global Biodiversity Framework, which was agreed in Montreal. And further work on the topic has been referred to q working group.
Read more details in our press release and see our pictures of the petition handover on Twitter.
Majority of European citizens rejects genetic engineering of wild species
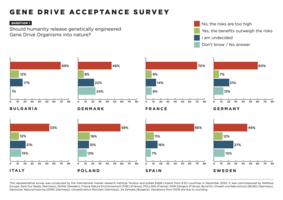
Should humanity release genetically engineered gene drive organisms into nature?
An alliance of European NGOs commissioned a representative opinion poll to determine how the European population evaluates gene drive technologyand how well known the issue is.The response of a majority of citizens in eight European countries is: “No, the risks are too high”. This first opinion poll on the subject shows high levels of opposition to (46% - 70%) and very low levels of support for (7% - 16%) the use of gene drive technology in the environment.
The survey of nearly 9,000 people is representative of 280 million EU citizens from eight EU countries. It was commissioned by nine NGOs demanding an informed and inclusive public debate and a global moratorium on the environmental release of this new type of genetically modified organisms. The survey also reveals that a large proportion of respondents were still undecided (14% - 27%) or did not know how to answer (1% - 24%). For more information on gene drives and all other results of the survey, please see the links below.
The Press release of the gene drive survey at EU level
To the full survey with all results here
14.02.2024 | permalink
EU Parliament disregards science by endorsing deregulation of new GM plants
After the EU Commission, the EU Parliament now also displays a clear disregard of science, by endorsing the deregulation of new genetically modified (GM) plants. It thereby puts EU citizens and the environment at risk, in conflict with the Parliament’s mandate to represent their interests. Citizens must now hope that the EU Council, which is still undecided, will stop this deregulation.
By a narrow majority (7%), the EU Parliament has endorsed the Commission’s proposal to deregulate GM plants made with New Genomic Techniques (NGTs), albeit with some amendments. The Parliament has proposed to maintain traceability and labelling of products of the plants (which the Commission wants to abolish) and to introduce a safeguard clause, meaning that a plant or product may be withdrawn from the market if a risk to health or the environment appears. However, the safety of NGT plants and products is still not guaranteed, as risk assessment remains absent from the proposal. So risks may materialise upon consumption or cultivation and may not be dealt with until they are discovered.
07.02.2024 | permalink
For a science-based regulation of plants from new genetic techniques
Technological progress makes genetic engineering a rapidly developing field. In its proposal of July 2023, the European Commission (EC) aims to deregulate a subset of new genetic techniques (NGT). This proposal would exempt certain NGT plants from the current EU regulatory framework for genetically modified organisms (GMOs) based on a considered equivalence with conventionally bred plants. Similar to the French ANSES, the German Federal Agency for Nature Conservation (BfN) argues in its new policy brief that this approach of considered equivalence lacks a valid scientific basis and violates the precautionary principle, since plausible risks cannot be excluded.
07.02.2024 | permalink
EU Parliament in favour for deregulation of NGT plants, BUT …
Contradictory results in Strasbourg
7 February 2024 / The EU Parliament (EP) today voted in favour of the deregulation of plants derived from new genetic engineering (NGT). It is doubtful that all MEPs have understood what they decided. A comment by Pascal Canfin on X (formerly Twitter), in which he claims that these plants would only be used to save pesticides and combat climate change, seems almost satirical. Canfin is chairman of the EP’s Environment Committee. He also suggested that a vote should be held first and only then should EFSA be asked for a further opinion on the risks.
06.02.2024 | permalink
New publication highlights the risks of NGT oilseed rape
Crucial questions on future regulation of NGT plants still unresolved
6 February 2024 / A new study, which has appeared as a 'preprint', highlights the environmental risks associated with the use of new genetic engineering (NGTs) in oilseed crops, such as rapeseed and camelina. These plants are by no means harmless in the environment: a frequently pursued goal is a change in the composition of the oil. However, both increasing and decreasing the polyunsaturated fatty acid content can have negative effects on pollinators feeding on the pollen of the NGT plants.
Furthermore, if NGT plants are introduced into agriculture, it is not unlikely that many different genetically engineered plants could be simultaneously released into the environment. Once released, the plants may interbreed with each other or with wildlife species, and thus spread in the environment.
31.01.2024 | permalink
Green Week 2024: Important commitments to freedom of choice regarding GMOs
The German Federal Agriculture Minister Cem Özdemir (Greens) and Peter Hauk (CDU), his regional counterpart from Baden-Württemberg, made an explicit commitment to maintaining freedom of choice for genetically modified food at the "Grüne Woche" [Green Week] 2024 in Berlin. VLOG used the trade fair for talks with politicians.
Özdemir: Extremely strong market needs to be protected
"Anyone who wants to farm GMO-free must be able to do so reliably in the future," said Özdemir in his opening speech at the reception of the organic food sector at the Green Week. "This is about an extremely strong market that has the right to be protected," added the Minister, "addressing those who otherwise always sing the praises of the market economy and market forces".








